What are alcohols?
Alcohols are a homologous series of chemicals. This means all alcohols have similar physical and chemical properties.
Alcohols are saturated organic molecules. They are saturated because they only contain single bonds so no additional atoms can be added to them. They are organic because they contain hydrogen and carbon atoms.
Examples of alcohols
Alcohols have the general formula CₙH₂ₙ₊₁OH (or CₙH₂ₙ₊₂O)
Ethanol is the most common alcohol it has the formula C₂H₅OH
Other alcohols all contain different numbers of carbon atoms.
Naming alcohols
The prefix of the name of an alcohol is dependant on the number of carbon atoms it contains. The suffix of an alcohol's name with always be -anol because it is an alcohol.
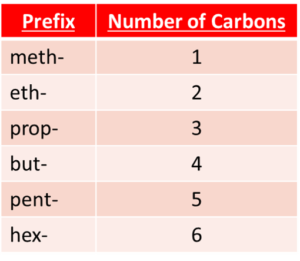
For example, an alcohol with four carbon atoms would have the name butanol
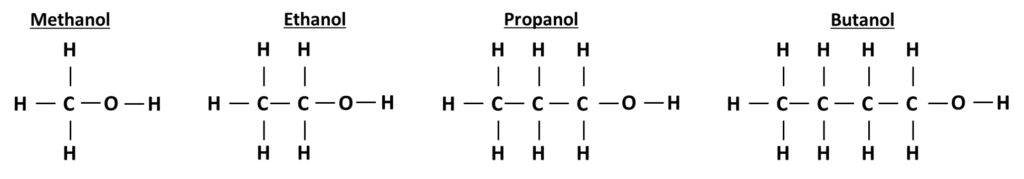
The structure of alcohols
All alcohols have a functional -OH group attached at the end of the alcohol. All carbon atoms are bonded to other carbon atoms with single bonds, all other bonds are single carbon - hydrogen bonds.
The properties of alcohols
All alcohols have the following properties
- Good solvents
- High combustibility
- High volatility
The production of alcohol
Alcohol can be produced in two different ways. Through the fermentation of glucose by yeast or through catalytic addition.
Fermentation of yeast
Yeast can turn glucose into ethanol through anaerobic respiration.
glucose --> ethanol + carbon dioxide (+energy)
C₆H₁₂O₆ (aq) --> 2C₂O₅OH (aq) + 2CO₂ (g)
Catalytic addition
Alcohol can also be produced by reacting alkenes with steam at high temperature and pressure while using a catalyst.
ethene + steam ⇌ ethanol
C₂H₄ (g) + H₂O (g) ⇌ C₂H₅OH (l)
If different alkenes are used in the reaction, different alcohols are produced, for example propene would produce propanol.
« Back to Glossary Index
